Dishwasher Filmology: Handling Hard Water and Mineral Buildup Problems in Today's Wimpy Dishwashers
Intro
The dishwashers being made today ain’t like yo mamma’s dishwasher. Dishwashers made as recently as 1997 used big honkin’ motors that practically sandblasted your dishes clean and then reversed direction to pump the water out. They used a lot more energy and water than the delicate little dainties being made today. They also lasted much longer and weren’t as prone to having problems with mineral buildup inside of ‘em… but don’t get me started on that rant. Prodded along by the Beltway Bandits wielding the Energy Star stick, all the manufacturers are making their dishwashers with dainty little wash motors that drizzle the water on the dishes and a separate drain pump motor. So, for better or for worser, we’re all stuck with these limp-wristed dishwashers.
One of the consequences of using these low-wattage pumps and motors is that they have to run longer to get your dishes as clean as the old war horses did. Whereas yo mamma’s dishwasher would run for less than an hour, it’s not unusual for a new dishwasher to run for two or three hours. Although it seems counterintuitive (that’s one of those big words that we professional appliantologists use– please don’t try it at home as I cannot be responsible for your safety), the newer dishwashers are still using less energy than the older ones even when they run two or three times longer. It’s madness, I tell you, unmitigated insanity!
But, like with everything, there’s a downside to all this feel-good, Energy Star madness. If you have hard water (like most folks who get their water out of a well), the reduced water use and longer run times mean you’re gonna get more mineral deposit gookus on your dishes and in the guts of your new fancy-pants, Energy Star dishwasher. This can cause all kinda washability and cleaning problems for your dishes, damage to the dishwasher’s dainty little innards, and increased energy consumption.
I get several calls a week for poor washing complaints in newer dishwashers. When I go out on these service calls, I often find that there's nothing wrong with the dishwasher from a repair standpoint. Yes, it doesn't help that all dishwashers made have been neutered by the Energy Star requirements which mandate that they use less water and electricity. And that's only going to get worse. It also doesn't help that phosphates were quietly removed from detergents by the manufacturers in July of 2010. You combine these two things with homes with hard water, like most people on a well, and you have the perfect storm for dishwashers that don't do a good job of washing the dishes. One common result is film left on dishes, especially evident on glassware.
Dishwashers need three things to do their job:
1. Mechanical action: water sprayed from the spray arms,
2. Thermal action: water heated to 120-125 deg. F to activate detergent,
3. Chemical action: good quality, fresh, dry POWDERED detergent matched to the amount of water hardness, and the use of rinse-aid.
If the dishwasher itself is operating within specs (conditions 1 and 2 above are satisfied) then almost all dishwasher washability complaints come down to the third condition: bad chemistry. Chemistry problems will involve one or more of these factors:
- Detergent
- Rinse Aid
- Water Hardness
In today's epistle, we're gonna look at all three of these factors in detail and'll reveal some esoteric techniques for dealing with dishwasher film and mineral accumulation problems in today's wimpy dishwashers using phosphate-free detergent in hard water environments. Whew!
Okay, grab 'hold of those two large lumps that you're sitting on and let's get ready to R-R-R-R-R-R-R-U-M-M-M-B-L-L-L-L-L-E!
Background Info: The Hard Facts About Hard Water
Complaints about the washer or dishwasher not cleaning very well are one of the more common ones I get from grasshoppers at my website and from paying grasshoppers in the field, also known as customers. The first thing I always like to figger out in these cases is how hard the water is. What’s water hardness and why’s it so ding-dang important? Well, Hoss, hang on ’cause I’m gonna ‘splain it to you.
Hardness is a term them fancy-pants engineers use to talk about dissolved minerals, mostly calcium and magnesium, in the water. Water picks it up as it flows in rivers or in aquifers under the ground, dontcha know. It varies from place to place according to the types of rocks you got.
Anyway, them fancy-pants engineers went on and created a way of measuring hardness. They call it grains per gallon, and you’ll see ‘em write it as "gpg." So, the more grains of calcium or magnesium dissolved in each gallon of water, the harder it is. Here's how they talk about hardness in numbers:
1 gpg = 1 grain per US gallon = 17.1 mg/l CaCO3 (US water hardness)
1 ppm = 1 mg/l CaCO3 (US)
1°e = 1 grain per UK gallon = 14.29 mg/l CaCO3 (English water hardness)
1°d = 10 mg/l CaO (German water hardness)
1°f = 10 mg/l CaCO3 (French water hardness)
1 gpg = 1.712°f
1 gpg = 0.959°d
1 gpg = 1.198°e (gpg UK)
1 gpg = 17.120 ppm CaCO3
So, what’s wrong with having a little calcium and magnesium in the water? Nothing if it’s just a little, like less than 3 gpg. But, when you start getting water with 7 gpg or more, you gots what we in the trade call, "hard water." And if it’s more than about 10 gpg, it’s called "damn hard water." This nifty little table summarized what I be sayin':
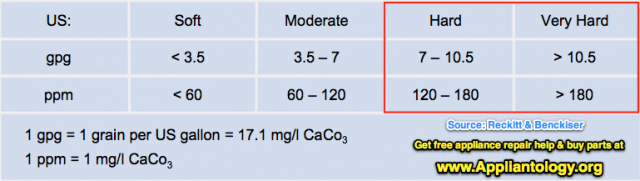
Y’see, Hoss, in a washer or dishwasher, these little calciums and magnesiums suck up the soap or detergent leaving less of it available to clean the crud off your clothes or dishes. As a result of water hardness, it takes more soap or detergent to get your clothes or dishes to an acceptable level of cleaness. Now you see why I always wanna find out what the water hardness is when I’m dealing with a poor cleaning complaint?
Now here’s something else to think about. The detergent instructions on the box are based on average hardness. Average hardness in the U.S. is defined as 6.6 gpg and 6.1 gpg in Canada. Now this cuts both ways, Hoss, hang with me for a minute. What if your actual water hardness is only 2 gpg but you’re putting detergent in your washer based on the directions on the box (typically a standard "scoop" or "capful")? Well, it don’t take a certifiable appliance guru like myself to figger out that you’re using too much freakin’ detergent! You’re not only wasting money, but you’re wearing lots o’ that stuff in your clothes, too.
Awwite, what if your actual water hardness was 15 gpg and you’re putting in detergent according to the amount on the box? Why, it means you are one dirty dude ’cause your clothes ain’t getting clean when you wash ‘em. Think about that next time you put on a pair of "clean" underwear.
Not only is hardness a problem from the standpoint of cleaning your stuff, it messes up your appliances, too. Oh sure, after a while, all kinds of "scale" from the hardness collects on the innards of your washer and dishwasher and screws it all up. But hey, I love the work it generates so I ain’t complainin’!
Now, the question you’re all axin’: "how do I measure the hardness of my water?" Piece of pie, tovarish. Come git you a Maytag Water Hardness Kit:
Dishwasher Detergents and Phosphates
The main tasks of a detergent are to remove soil from surfaces and prevent the re-deposits of soils on the surfaces. The best detergent formulations will be powdered. Do not use gels or liquid detergents.
Why powdered detergent? Because in today's phosphate-free world, you need two types of cleaners in a detergent formulation to get dishes clean:
1. Enzymes to remove protein-based stains
2. Bleach to remove other stains
These two cleaners are incompatible with each other-- if they're released at the same time, the bleach will destroy the enzyme and, after this epic battle, there will be little or nothing left of the bleach to do even its little bit of cleaning. The result: dirty dishes. They can coexist in a powdered form because they are not activated until 1) they get wet and 2) the water temperature reaches 125 deg. F. In a liquid or gel form, everything is already wet so you're only getting one kind of cleaning action.
Detergent has a shelf life. Old detergent will not work well because the enzymes denature over time. Also, the detergent must stay dry until it's time to use it. Once it gets wet or even damp, it activates and will no longer be active when put to work inside the dishwasher.
It is not only okay to put dirty dishes into a dishwasher, it is mandatory to properly activate the detergent. Detergents are designed to work with food soils, not clean water. Without the food soils, the detergent will create a caustic slurry inside the dishwasher which will etch the glassware by removing the silica from the glass.
Not only that, but pre-rinsing the dishes wastes water. DOE estimates that pre-rinsing dishes uses 20 gallons of water per load. Scrape the chunks off with a fork and leave the rest on the dishes. It's a dishwasher, for crying out loud! Let it do what it was designed to do!
Let's look at the detergent ingredients in action:
- Alkalies regulate the pH of the cleaning solution to help remove soils from the surfaces
- Builders regulate water hardness and prevent the formation of limescale deposits
- The bleach system eliminates certain soils (e.g. tea and coffee stains) by oxidizing them and making them water removable
- Enzymes work as catalysts and, in general, work best at temperatures around 122°F. Protease breaks down protein-based stains such as egg. Amylase breaks down starch-based stains such as oatmeal and potato.
Phosphates did a number of important things to help the detergent clean better. For example, phosphate causes food to break apart and dissolve by removing the calcium that binds foods together. It also reduces spotting and filming during the wash cycle. Phosphate also helps break up and get rid of grease, helps control water hardness, and suspends soils within the wash water so they are not redistributed onto the plates.
The old-formula dishwashing detergents had about 30% phosphates. Now, with the phosphates removed, the calcium is free to run around inside the dishwasher slurry causing trouble, a particular problem in hard water areas. But the Samurai has a few tricks for dealing with this situation that you can share with your customers.
The detergent manufacturers all quietly and "voluntarily" removed phosphates (as tri-sodium phosphate, or TSP) from detergents on July 1, 2010. I say "voluntarily" because they did this to pre-empt a whole new riot act of regulations about to come out under the Clean Water Act which would have had more far-reaching effects and, as regulations always do, make detergents even less effective and more expensive. So the industry did damage control by voluntarily removing TSP from the detergents.
This in itself caused a rash of washability complaints. All of sudden, the same type and amount of detergent that people had been using for years in their dishwasher no longer did the job. I remember being inundated with dishwasher complaints through the rest of 2010.
How to Handle Hard Water and Mineral Buildup in Today’s Wimpy Dishwashers: Epilogue and How-to Guide
Contrary to popular belief, you do not want the water to be too hot in hard water conditions. If you use any of the water heating options (Sani, Temp boost, etc.) and you're having filming problems on the dishes and glasses, try disabling those options. If you are in a hard water area, you can also get flash drying, which means the water will evaporate off the glasses and plates instead of rinsing off, leaving behind grit and all the hard water deposits. You want the water to be at least 125℉ or so to activate the detergent, but that’s about it. Use a good POWDER detergent. Recommended amount I believe is 1 tablespoon per grain of hardness; if you are 10 or more, fill the cups and boost your rinse aid up to Max.
I describe flash drying to people with this scenario. If you had a gorgeous black car, would you wash and wax it in the heat of the day in direct sun? When they say "no" I ask them "why" and they state it would leave white spots all over it. This is what happens in the dishwasher. Glasses look like they were rinsed in milk, and fine food particles are left behind. Even the dishwasher itself can get all gunktified like ahso:

To prove your glasses have hard water filming (can be cleaned) and are not etched (irreversible damage) try filling one up with white vinegar half way and let it soak. If when you rinse it the whiteness is gone from the area where the vinegar was, it’s hard water filming. Let's watch it in action:
So let's end with four practical things you can recommend to your customers having dishwasher cleaning problems:
Numero Uno: Use rinse aid! It’s not an option with today’s dinky dishwashers. Rinse aid allows the dishwasher to use less water with the same amount of cleaning and drying effectiveness. It does this by creating what we professional appliantologists call “sheeting action” of the water. By making the water sheet along dishes, rather than cluster into beads, it evaporates faster and with less energy. Look at the difference:

Numero Duo: If a little Rinse Aid doesn’t work, use MORE!

Numero Trio: Regularly use a dishwasher cleaner (Affresh) and performance booster (Glass Magic) to clear out the gookus and reduce the build-up.
Numero Quattro: Install a household water softening system or buy a fancy-pants dishwasher with its own water softener built in, like a Miele.
-
.png) 2
2








3 Comments
Recommended Comments